A Vietnamese company based in Ho Chi Minh City which makes drugs for the cure of Hepatitis B, has for the first time in Vietnam produced a drug for Hepatitis C in an injection form.
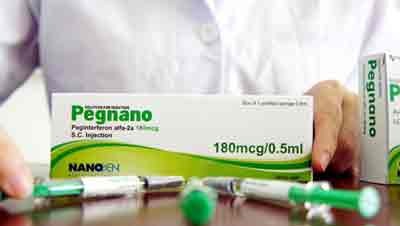
However, the Vietnamese company Nanogen Biopharmaceutical Ltd faces a lawsuit after the new medication was recently registered by authorities.
Freshview Intellectual Property Law Company in Hanoi, a representative of F.Hoffmann-La Roche AG in Vietnam has filed a complaint in a letter to the Nanogen Biopharmaceutical Ltd Company accusing the company of violating the patent protection law under which Roche is sole owner of Peginterferon alfa 2a the drug they have patented in Vietnam.
Roche claims the patent No. 2611 for the mentioned drug was granted by the National Office of Intellectual Property of Vietnam in 2002 and is valid until May 2017. Therefore no company in Vietnam can manufacture the interferon compound without permission from Roche.
Roche affirmed that the Nanogen Company was violating their patent rights and requested the company to immediately stop producing, marketing and advertising its Pegnano drug from December 30.
Roche also demanded the registration of Pegnano drugs be revoked by the relevant authorities.
On September 30, Truong Quoc Cuong, head of the Drug Administration of Vietnam, a division of the Ministry of Health turned down Nanogen’s request to register and sell its Pegnano drug after Roche protested.
However, Cao Minh Quang, deputy health minister, authorized the registration of three products on December 8 as Nanogen Company assured him that it had not violated the patent law.
In a talk with Tuoi Tre newspaper, Ho Nhan the Nanogen Company director denied that the company had violated the patent law stating that he had respected all intellectual property rights.
According to Mr. Nhan, Pegnano is a hi tech product which the Vietnamese government had encouraged and supported.

Moreover, the company had dedicated 9 years to making the drug accessible and within reach for poor Vietnamese hepatitis patients. Pegnano is sold for VND1.5 million-1.9 million per 180mcg (USD77) compared to Roche’s selling price of VND4.3 million for the same amount.
Mr. Nhan cited article 7 of the Vietnamese Intellectual Property Law which was revised in 2009 where it is clearly stated that the government can ban or limit the exercise of intellectual rights in cases where such rights harm national defense, welfare of the people or affects other crucial national interests.
Dr. Tran Tinh Hien, deputy director of the Ho Chi Minh City based Tropical Disease Hospital supported Pegnano manufacture in Vietnam as he believed that many countries would be willing to revoke intellectual patent rights in order to have cheap HIV medicine.
Hoang Huu Doan, former director of the state-run Central Pharmaceutical Factory No. 1 has also backed Pegnano as he believed that drug manufacturing monopoly must end.