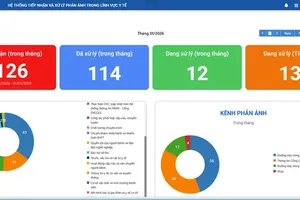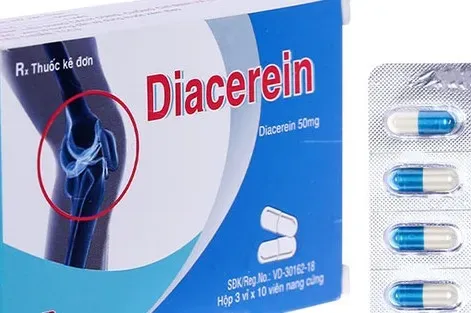
The recalled batch of Diacerin 50 was issued a marketing authorization certificate under registration number 893110447024, batch number 0125, with a manufacturing date of April 20, 2025, and an expiry date of April 20, 2028. The product was manufactured by the Thanh Hoa–based branch of the Joint Stock Company for Pharmaceuticals and Medical Biology, located in Hac Thanh Ward. Diacerin 50 is prescribed for the treatment of certain musculoskeletal and joint-related conditions.
The Drug Administration has instructed the branch of the Medical Biomaterial and Pharmaceutical Joint Stock Company to immediately suspend the distribution of the affected batch and place any remaining stock under special storage. The company is required to report on the product’s distribution and to take the lead, in coordination with relevant organizations and individuals, in issuing recall notices to all wholesalers, retailers, pharmacy chains, medical facilities, and end users who have received the recalled batch.
Members of the public who are currently using the affected batch have been advised to discontinue use immediately and return the product to the dispensing facility.