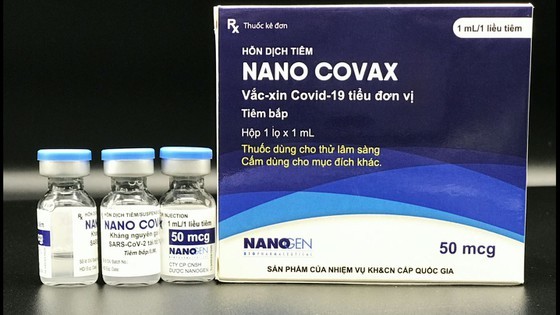
 Vietnamese-made Covid-19 vaccine Nanocovax has been approved by the National Ethics Committee in Biomedical Research.
Vietnamese-made Covid-19 vaccine Nanocovax has been approved by the National Ethics Committee in Biomedical Research.
All dossiers and data presenting information about the search for Nanocovax vaccine have been submitted to the MoH’s Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients for emergency use authorization.
The vaccine is expected to be got the circulation license granted by the Drug Administration of Vietnam under MoH within 20 days of receiving the application.
Last week, the National Ethics Committee in Biomedical Research hold a meeting on assessment of clinical trial reports of the phase 3a mid-term of Nanocovax and evaluation of its safety and the generation of immune response.
According to the live virus neutralization assay, the vaccine has achieved a 96.5 percent efficacy at the 42nd day (14 days after second shot).
On 42th day of injection, the antibody level of Nanocovax has increased by 218.93 times and its seroconversion rate is 99.2 percent. The study suggested that the vaccine was approximately 75 percent effective against preventing infection from the Delta variant.
Nanocovax Covid-19 vaccine is produced by Nanogen Pharmaceutical Biotechnology JSC based on recombinant DNA/protein technology.