 Made-in-Vietnam Covid-19 vaccine yields good result
Made-in-Vietnam Covid-19 vaccine yields good result
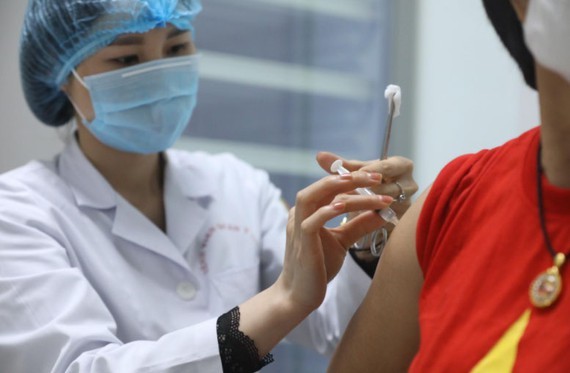
Due to the urgent demand in the fight against Covid- 19, the National Council for Ethics in Biomedical Research has allowed the third phase trial of the Nano Covax vaccine to evaluate its trial results. Around 13,000 volunteers get jabs in phase 3 of human trials of home-grown Nano Covax vaccine to evaluate its effectiveness.
Associate Professor Ho Anh Son, Deputy Director of the university’s Institute of Biomedicine and Pharmacy revealed the Military Medical Academy and the Pasteur Institute in Ho Chi Minh City each will inject 500 volunteers in the early time of the third phase of the NanoCovax vaccine trial while 12,000 volunteers will receive their second shot later.
As per schedule, by mid-September, after about 42 days of the second injection of the first 1,000 volunteers, related parties will evaluate the vaccine effectiveness. If the result of the trial is good, they will submit a document to the Government, the Ministry of Health, and the National Council for Ethics in Biomedical Research for approval.
Director of the Military Medical Academy Prof. Dr. Do Quyet said that the prior trials showed the vaccine was safe and vaccinated volunteers had antibodies against the coronavirus.
The Ministry of Health also announced the second trial of the COVIVAC vaccine developed by the Nha Trang Institute of Vaccines and Biologicals will be conducted in the Northern Province of Thai Binh adding that the Nha Trang Institute of Vaccines and Biologicals can make about 6 million doses of COVIVAC vaccine a year.
The Ministry of Health has established a steering committee to help and support domestic producers in Covid-19 vaccine technology transfer. One Covid-19 vaccine producer has discussed and negotiated with a manufacturer in the United States about technology transfer for the production of the Covid-19 vaccine from mRNA essence. mRNA Covid-19 vaccine effectiveness against SARS-CoV-2 infection is high for full immunization with one dose. This producer can make 100-200 million doses a year, and it is expected to start production from the fourth quarter of 2021 or the first quarter of 2022.
Company for Vaccine and Biological Production No.1 (Vabiotech) has been negotiating with a Japanese partner to soon receive the transfer of vaccine production technology. Vabiotech also signed an agreement with the Direct Investment Fund of the Russian Federation on the packaging of Covid-19 vaccine tubes Sputnik-V from semi-finished products with a scale of 5 million doses a month, starting from July 2021. The company orientates towards production technology transfer of 100 million doses annually.
Vietnamese Ministry of Health has approved the emergency use of Covid-19 vaccine Comirnaty developed by U.S.-based firm Pfizer .