 The Ministry of Health states that Protamine sulfate is a drug on the list of rare drugs that should be given priority in consideration for the issuance of a circulation registration certificate
The Ministry of Health states that Protamine sulfate is a drug on the list of rare drugs that should be given priority in consideration for the issuance of a circulation registration certificate
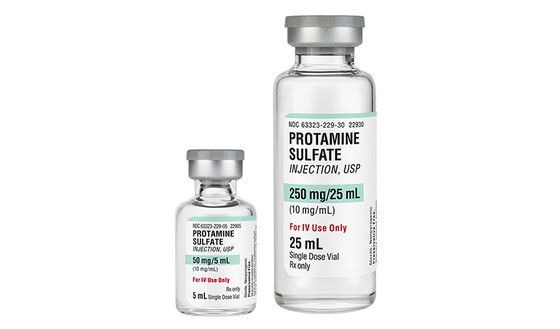
Accordingly, the Ministry of Health stated that Protamine sulfate is a drug on the list of rare drugs that should be given priority in consideration for the issuance of a circulation registration certificate; however, no facility has so far submitted an application for the circulation registration of medicines with the active ingredient Protamine sulfate in Vietnam.
Recently, the Drug Administration of Vietnam has licensed many establishments to import injectable drugs containing the active ingredient Protamine sulfate (which has not yet been granted a circulation registration certificate) to meet the special treatment needs of medical examination and treatment establishments. according to the provisions of Article 68 of the Government’s Decree No. 54/2017/ND-CP detailing several articles and measures to implement the Law on Pharmacy.
According to information from importers, although the quantity of Protamine sulfate drugs that have been licensed for import is exactly the amount estimated by medical examination and treatment establishments in need, the quantity of Protamine sulfate drugs imported into Vietnam is still short in the near future as the demand is higher than the current estimate.
The main reason for this is that Protamine sulfate is a specialized drug, only used in cardiac - thoracic surgery procedures with not much demand. Therefore, manufacturers usually only produce after receiving orders from other facilities.
Meanwhile, after receiving estimates from medical examination and treatment facilities and receiving import permits from the Ministry of Health, Vietnamese importers will place orders with foreign drug suppliers.
Therefore, in case medical examination and treatment facilities do not proactively and promptly place orders with drug importers and from drug importers with foreign drug suppliers, it may lead to a temporary shortage of drugs when Vietnamese importers place an order but foreign drug suppliers do not have enough stock to supply as required for the Vietnamese market. It will take a few months for manufacturing new drugs.
The Ministry of Health also said that because Protamine sulfate is a rare drug supply and demand are lower than other drugs; therefore, in recent years, the Drug Administration of Vietnam has sent dispatches to health departments, hospitals, medical institutes, and importing establishments requesting to proactively contact the importing companies of Protamine sulfate medicine to place orders.
Last but not least, health departments, hospitals, medical institutes, and importing establishments should stockpile the drug beyond regular demand in case of emergencies, sudden demand, or problems in drug supply in addition to the regular quantity. These bodies should review their ordering, purchasing, and stocking injectable drugs containing Protamine sulfate active ingredient to avoid interruption of drug supply for the hospital's treatment needs.
With the current situation, the Drug Administration of Vietnam requires importing establishments of Protamine sulfate drugs to urgently synthesize all estimates of medical examination and treatment establishments to plan and sign contracts early with establishments. Foreign drug manufacturers and suppliers to ensure that these establishments can be proactive in producing and supplying drugs in a timely manner to the Vietnamese market, avoiding drug shortages due to late signing of contracts.
Moreover, they should work with medical establishments and infirmaries in desperate need of the drug to place orders for Protamine sulfate drugs in accordance with regulations and submit them to the Drug Administration of Vietnam immediately after finishing the files.
Because this is an indispensable item in the heart - thoracic surgery process, to ensure a timely and sufficient supply of drugs for use, the Drug Administration of Vietnam recommends that the health departments of the provinces and cities, hospitals, institutes, and drug-importing establishments seriously implement the directing documents of the Drug Administration of Vietnam related to the supply of Protamine sulfate drugs that have been issued in the past time.
Drug import establishments shall report to the Drug Administration of Vietnam before August 20 on the plan to import Protamine sulfate in the near future and propose solutions to overcome.