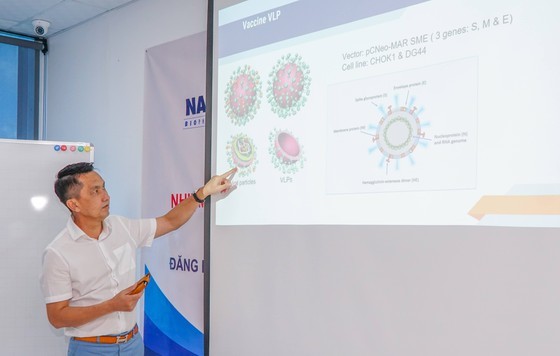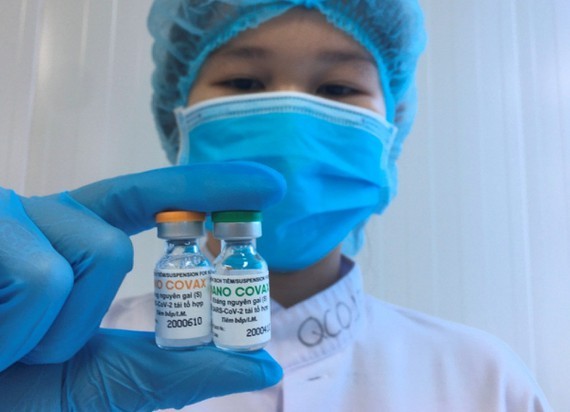
As the spread of the virus is likely to continue disrupting economic activity and negatively impact the country’s manufacturing and service industries, the Nanogen Pharmaceutical Biotechnology has developed the NanoCovax vaccine to halt the spread of Covid-19.
The results of the trial’s second phase showed that the Nancovax vaccine was better than expected; therefore, the National Council of Medical Research Ethics and the Ministry of Health has approved the third phase of the clinical trial on 13,000 volunteers. A trial showed that the NanoCovax coronavirus vaccine had an efficacy rate of 99,4 percent plus, its price is just VND120,000 (US$5.22) a dose.
Nanogen said its current production capacity is 20 million doses to 12 million doses a month. Moreover, it has expanded its cold storage to contain 10 million doses of vaccine. Accordingly, it is expected to supply 50 million doses from now to December 2021 and 100 million doses of vaccine by 2022.
 Researchers are making the vaccine
Researchers are making the vaccine
With its determination to achieve herd immunity by the second quarter of 2022, the company petitioned for emergency authorization for the vaccine like other vaccines.
Previously, on June 11, the Ministry of Health issued Decision No. 2899 approving the phase 3 clinical trial of the NanoCovax vaccine. The clinical trials were conducted at the Military Medical Academy and the Pasteur Institute in Ho Chi Minh City; deployment locations at Military Medical Academy in the Northern Province of Hung Yen, the Ho Chi Minh City-based Pasteur Institute, in the Mekong Delta provinces of Long An and Tien Giang and some other venues.
The Ministry of Health documented an additional 100 domestically- transmitted Covid-19 cases on Tuesday afternoon, raising the national tally to 13,630 with 69 deaths.