 Illustrative photo ( Photo: SGGP)
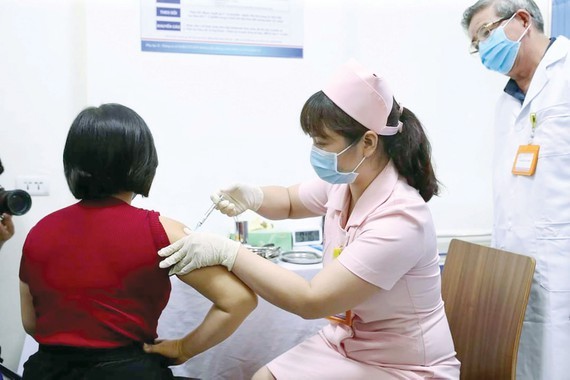
Illustrative photo ( Photo: SGGP) According to Director of the Clinical Medicine Center Pham Thi Van Anh, the center conducted the trial on 15 volunteers only who had to stay in the center for further observation. Volunteers are people aged from 18 to 59. As scheduled, 15 other volunteers will receive first shots of the vaccine on March 25.
Beforehand, a group comprising of six volunteers were also administered the shots of Covivac vaccine on March 15.
Nguyen Ngo Quang, Deputy Director of the Department of Science, Technology and Training under the Ministry of Health, said that the trial’s first phase of the Covivac vaccine has gone through, pre-clinical researches have shown good results. Moreover, the price of the vaccine is cheaper than available vaccine in the international market.
Mr. Quang added that Vietnam has four research institutions working on Covid-19 vaccines including the Vaccine and Biological Production No.1 (Vabiotech), the Vietnam's Center for Research and Production of Vaccines and Biology (Polyvac), the Institute of Vaccines and Medical Biologicals (Ivac), and the Nanogen Pharmaceutical Biotechnology. Vietnam has been mulling human trials of Covid-19 vaccine. NanoCovax vaccine developed by Nanogen Pharmaceutical Biotechnology has ended its human trial’s first phase and most of its volunteers are safe without serious allergic reactions. Plus, test results have shown that the vaccine has proven to generate high immune response and to be effective against the novel coronavirus, even its new variant first reported by the UK health authorities.
If everything goes smoothly, there will be very high likelihood that the trial’s third phase of the NanoCovax vaccine will be finished in the third quarter this year, Mr. Quang stressed. If the third phase can be completed in the third quarter of the year, Vietnam will use first Vietnamese-made vaccine against Covid-19.
In regard to enterprises’ proposal to purchase foreign-made vaccines, Health Minister Nguyen Thanh Long asserted that according to the government’s resolution 21/NQ-CP on purchase and use of Covid-19 vaccine, the Ministry and related competent agencies were assigned to buy and manage sponsored vaccine; accordingly, the Ministry has not allowed private companies to buy Covid-19 vaccine in the international market.
So far, the Ministry has contacted with vaccine manufacturers in the world. Only the Ministry-approved enterprises can import vaccine; however, administration of vaccine is still under the Ministry’s coordination following the government’s resolution 21/NQ-CP.
Covid-19 vaccine inoculation must be conducted in the health facilities which have been certified before. At a press brief, Dr. Tran Dac Phu, former Head of the Department of Preventive Medicine, said that Covid-19 vaccines in the world are newly-made kind; hence, good preparations for immunization including preservation of the vaccine, screening and post-vaccination observation are needed for receivers’ safety.
Also on the day, Deputy Health Minister Truong Quoc Cuong approved Russia’s Covid-19 vaccine, also known as Sputnik V, for emergency use in Covid-19 prevention and control in the country.