The vaccine was piloted in Binh Dinh Province on December 25, 26 and 27 but 27 kids were rushed to hospital in the first day; eight were hospitalized the next day and five in the third day.
Most of them were suffering high fever including five in serious condition and turned pale and had breathing problem, said Mr. Hung.
Fortunately, they all were discharged.
According to Mr. Hung, this is the second pilot vaccination in the province as per the Ministry of Health’s direction.
The first vaccination was in October, 2018 on 970 kids. Sixty of them had high fever and three serious cases.
Consequently, Binh Dinh halted vaccination to await the Ministry’s direction.
After one-month investigation, the Ministry requested to continue vaccination in Binh Dinh Province.
This time, there were no severe cases like in October because the provincial health sector prepared well, said Mr. Hung; all slight or severe cases must be under treatment.
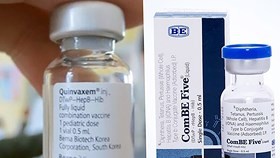
Produced by Indian vaccine manufacturer Biological E. Ltd., five-in-one pentavalent vaccine ComBE Five, that can help fight against five common, potentially fatal diseases affecting infants - diphtheria, tetanus, whooping cough, hepatitis B and Haemophilus influenza type B, was used nationwide to replace Quinvaxem from late December after it was tested in seven provinces.
Not only Binh Dinh but also other provinces such as Nam Dinh, Thai Binh, Ha Nam, Quang Ngai, Kon Tum, Ba Ria - Vung Tau have reported cases of hospitalized children after vaccination.