 |
Intravenous phenobarbital for HFMD treatment is re-supplied |
The Department of Health of Ho Chi Minh City today announced that 21,000 ampoules of intravenous phenobarbital have just arrived in Vietnam after a long interruption, promptly replenishing the drug source for the treatment of severe hand, foot and mouth disease for the three pediatric hospitals and the Hospital for Tropical Diseases in the city. Previously, the Department of Health of Ho Chi Minh City also received 1,000 vials of Gamma-globulin for the treatment of HFMD.
According to the HCMC Department of Health, at the end of 2020, the domestic supply of Phenobarbital was interrupted for a long time. The department's expert council met to come to an agreement to select other drugs on the market such as Diazepam and Midazolam for temporary substitutes while waiting for Vietnamese pharmaceutical enterprises to find sourcing from other countries.
At the same time, the Department of Health has also sent a written request to the Ministry of Health’s Drug Administration of Vietnam to ask for assistance in finding the supply of the special drug and importing companies to provide phenobarbital for the increasing treatment needs of hospitals.
Previously, on June 22, the Drug Administration of Vietnam was informed that the manufacturer Daihan Pharm Company did not continue to produce the drug Danotan; subsequently, the Department sent a written request to pharmaceutical importers to contact foreign partners for seeking the source of alternative medicine.
By February 1, 2023, the Central Pharmaceutical CPC1 company reported finding a supplier of Phenobarbital to replace it; thus, it was granted a drug import license by the drug authority for 21,000 ampoules of Barbit injection 200mg/ml from the Bangladesh manufacturer Incepta Pharmaceutical Company to Vietnam.
Because Phenobarbital is a psychotropic drug belonging to a group of drugs under special control, an export license is required from the competent authority of the exporting country. Until July 31, after a long time of carrying out formalities, some 21,000 phenobarbital ampoules arrived in Vietnam and are immediately distributed to Children's Hospitals in Ho Chi Minh City and the Hospital for Tropical Diseases.
Phenobarbital is recommended in the Ministry of Health’s treatment protocol for hand, foot and mouth disease in Decision No. 1003/QD-BYT dated March 30, 2012. According to the Ministry’s treatment protocol, Phenobarbital can be administered orally or by injection, preferably the injectable form when the patient is seriously ill. Phenobarbital is used for children with many advantages, few side effects, and has been used by doctors for a long time in pediatric patients.
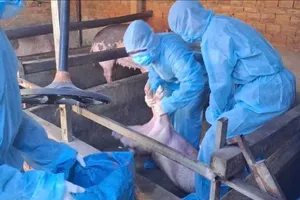
According to the Department of Health of Ho Chi Minh City, from the beginning of 2023 to now, the southern largest city has had more than 15,753 cases of HFMD with hundreds of new cases every day. Worse, many serious and critical cases were transferred from other localities to children's hospitals in HCMC. Amongst critical cases, most of the cases are children under 6 years old.
To proactively supply domestic supply, the Drug Administration of Vietnam has licensed the import of Phenobarbital raw materials to Danapha Pharmaceutical Company to manufacture Phenobarbital. This company is making the production plan.