This move is part of a broader campaign targeting smuggling, trade fraud, counterfeit goods, and intellectual property violations, as directed by the Prime Minister during a nationwide enforcement drive.
According to the HCMC Department of Health, joint inspection teams have examined 1,285 facilities involved in pharmaceuticals, medical equipment, cosmetics, and healthcare services. The Department itself directly inspected 226 establishments, including six cosmetics businesses, 80 pharmaceutical outlets, 121 medical device enterprises, and 19 healthcare facilities. Violations were found at 41 of these, resulting in fines exceeding VND2.2 billion (around US$86,000) and the confiscation of unlawfully obtained goods valued at over VND39 million.
Separately, the Department’s special task force penalized three additional violators with total fines surpassing VND572 million. Most infractions involved false advertising and the sale of goods with unclear origin or lacking proper documentation.
At the same time, health offices in districts and Thu Duc City conducted inspections at 1,059 medical and pharmaceutical practice sites, sanctioning 14 facilities with combined penalties of more than VND169.5 million.
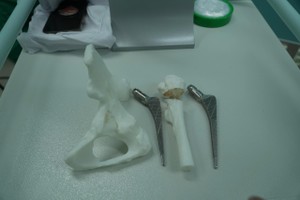
Notably, in the area of medical devices, the HCMC Department of Health issued 17 decisions to revoke a total of 364 product declarations due to documentation errors and improper device classification. The revoked declarations included products such as hand sanitizers, blood glucose meters, massage devices, feminine hygiene solutions, and ENT care instruments.
The Department observed a concerning trend: some businesses deliberately downplayed the risk classification of medical devices to facilitate registration and gain access to public procurement. Common violations included mislabeling non-medical products as medical devices, incorrect or inappropriate risk classifications, and incomplete or inaccurate self-declared documentation.
Although no counterfeit medical devices have been found in public hospitals or healthcare centers, the Department warned of potential risks associated with improperly imported or manufactured medical products.
Looking ahead, the HCMC Department of Health plans to tighten oversight of medical device self-declarations, restructure its professional advisory council, and enhance training efforts. It will also deploy information technology and artificial intelligence in regulatory and post-market surveillance. The Department emphasized that it will strictly handle any deliberate attempts to manipulate device classifications, in order to safeguard the interests of the public and legitimate businesses.