A Singapore-based company, Tychan, will start human clinical trials for a treatment that could slow down the progression of COVID-19 in patients, help them recover faster, and provide temporary protection against the coronavirus.
Tychan has developed TY027, a monoclonal antibody that specifically targets SARS-CoV-2, the coronavirus that causes COVID-19. Monoclonal antibodies can be isolated and manufactured in large quantities to treat diseases. Presently, there is no proven antibody-based treatment for COVID-19. There is also no licensed vaccine to prevent SARS-CoV-2 infection.
“You could use it to treat all COVID-19 patients and prevent them from getting severe disease. You could also give it to those who are going to get severe disease and prevent them then from sliding further in their respiratory function,” said Professor Ooi Eng Eong of Duke-National University of Singapore (Duke-NUS) Medical School.
For those who already require oxygen, the hope is that the drug will prevent them from needing a ventilator, and for patients who are already on ventilators, that they could go off the ventilators.
The drug will also be evaluated for its potential to provide temporary protection against infection with SARS-CoV-2. “We could even, for instance, give this to healthcare workers who are treating COVID-19 patients so that they don't get infections themselves,” Prof Ooi said, adding that this would depend on the results of the trial.
People traveling to places with many COVID-19 cases could also use the drug to prevent infection, he said.
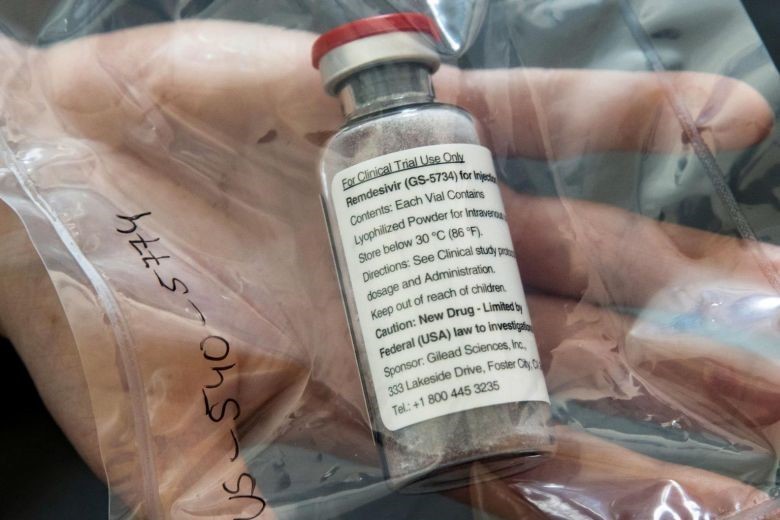
Another positive news from the local medical community is the conditional approval for antiviral drug, Remdesivir, to be used to treat seriously ill COVID-19 patients in Singapore, making the country one of the first to get the nod for using the drug to treat the virus. The drug, which was first created to treat Ebola, has been used as part of clinical trials in Singapore for COVID-19 patients.
The approval means doctors can now use Remdesivir to treat adult COVID-19 patients who require supplemental oxygen or require more intensive breathing support, such as the use of ventilators or life support machines. Patients who have oxygen saturation levels of 94 percent or less can also be given the antiviral drug.
























