According to Novartis Vietnam, three eye drop products are suspected to be falsified: Tobrex 5ml (batch numbers VEE98C and VEE90A), Maxitrol 5ml (batch number VFD09A), and TobraDex 5ml (batch number VHN07A).
The Drug Administration has instructed provincial health departments to notify businesses and the public not to purchase or use the above-mentioned products. It also requested Novartis Vietnam to provide detailed information and cooperate in tracing the origins of the suspect batches when required.
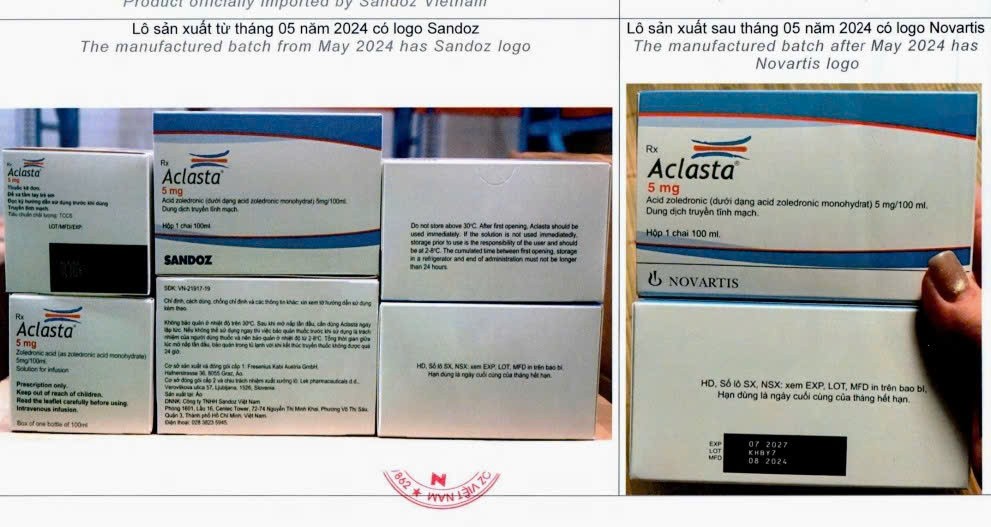
In a separate report to the Ministry of Health, Sandoz Vietnam Co., Ltd. reported the detection of a potentially counterfeit batch of Aclasta (a drug for osteoporosis and Paget’s disease of bone), batch number KHBY7, manufactured in August 2024 with an expiry date of July 2027. This batch was neither produced by Lek Pharmaceuticals d.d. (Slovenia) nor imported by the company. Notably, its packaging still carries the old logo, which has been discontinued since May 2024.
Meanwhile, the Drug Administration also requested the HCMC Department of Health to urgently verify and trace the origins of a suspect batch of Lexomil 6mg (a sedative), batch number F3193F01, expiring December 2027, after it was seized by HCMC police. Investigations confirmed that Lexomil 6mg was manufactured for circulation in France, has no marketing authorization in Vietnam, and has not been legally imported.
Authorities are continuing their investigation to clarify the matter and will handle violations in accordance with regulations.