Following the recent crackdown by Hanoi City Police on a ring producing counterfeit functional foods and medical equipment—resulting in the seizure of over 100 tons of fake goods—Deputy Director Ta Manh Hung of the Drug Administration of Vietnam under the Ministry of Health stated yesterday that, upon receiving news of the incident, the department immediately issued an urgent dispatch to relevant authorities requesting a comprehensive inspection report.
Addressing concerns that some of the counterfeit products had infiltrated hospitals, Deputy Director Ta Manh Hung explained that the regulation of pharmaceuticals is highly stringent, making it difficult for counterfeit drugs to penetrate hospital pharmacies.
Under current regulations, for a pharmaceutical product to be admitted into hospitals, the bidding entity must submit a capacity profile and a technical dossier for the drug. Additionally, the bidder must hold a valid certificate of eligibility for pharmaceutical business and be registered on the national bidding system.
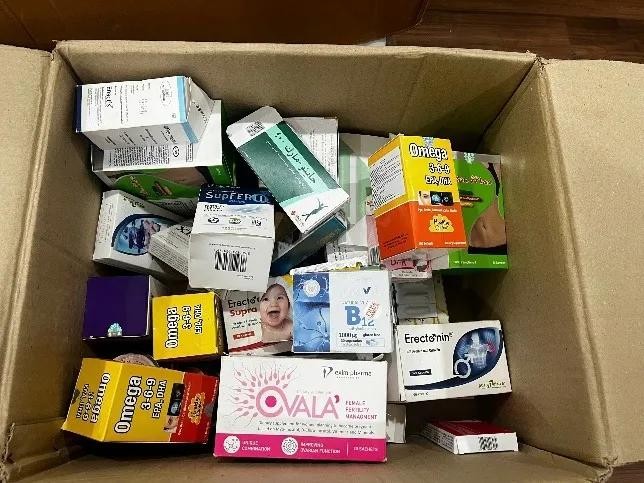
In contrast, counterfeit functional foods and medical equipment are more susceptible to entering hospital supply chains due to less rigorous regulatory oversight compared to pharmaceuticals. To address this gap, the Ministry of Health is currently reviewing and proposing amendments to Decree 15/2018/ND-CP, which guides the implementation of the Law on Food Safety, aiming to strengthen oversight and ensure the safety and quality of these products.
On May 16, the Hanoi Economic Police Department dismantled a criminal ring engaged in the production and distribution of counterfeit food and medical equipment, led by Pham Ngoc Tien and his wife, Doan Thi Nguyet, residents of Ha Dong District, Hanoi. Authorities seized over 100 tons of counterfeit goods during the operation.
In response, the Ministry of Health sent a document to people’s committees in provinces and cities, launching a peak campaign against smuggling, trade fraud, counterfeit goods, and intellectual property violations. The Ministry urged local authorities to intensify efforts to combat the production and trade of counterfeit or unidentified drugs, health supplements, and milk products. It also emphasized the importance of receiving and acting on reports and denunciations of related crimes, as well as overseeing conditional business practices within the health sector.
Additionally, the Ministry called on local health departments to coordinate with the Department of Science and Technology and other relevant agencies to promote responsible communication. Scientists and health administrators are urged to sign commitments refraining from advertising or endorsing products with misleading, unscientific, or exaggerated claims without verified legal documentation. Violators will be held legally accountable for any false advertising activities.
Separately, the Ho Chi Minh City Department of Health issued an urgent notice to medical institutions and pharmaceutical businesses, requiring immediate review and action to detect and prevent the circulation of counterfeit drugs, functional foods, and medical equipment in the market.