 |
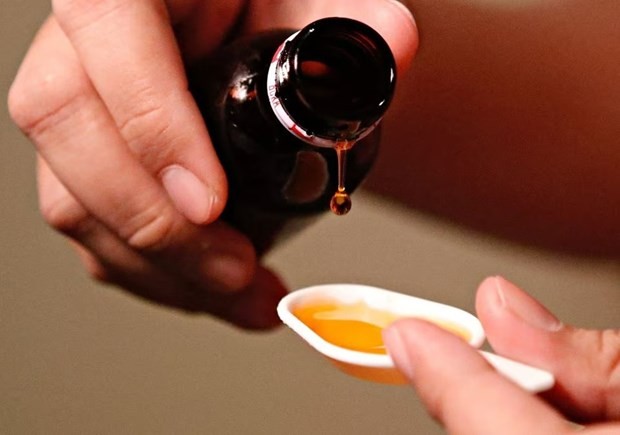
The cough syrup products have caused death or acute kidney damage for hundreds of children.
Previously, the ministry received a relevant warning from the International Criminal Police Organization (Interpol) that these products, produced in India and Indonesia, contain diethylene that can cause serious harm to health or death to users.
According to the Drug Administration of Vietnam under the MoH, these products have not been granted drug registration certificates in Vietnam and certificates for import into the country.
To ensure safety for users, the ministry asked health departments of provinces and centrally-run cities, and medical establishments under the MoH to urgently disseminate information related to the serious harm of these products.
Health departments have been requested to conduct inspections at pharmaceutical business establishments on the circulation of these products in particular; medicines without origin, or those that have yet to be approved for circulation, in general.
Meanwhile, health facilities nationwide have been also demanded to not use the syrups, which are listed as follows.
Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup produced by Maiden Pharmaceuticals Limited; Temorex Syrup by PT Konimex; Flurin DMP Syrup by PT Yarindo; Unibebi Cough Syrup, Unibebi Demam Paracetamol Drops and Unibebi Demam Paracetamol Syrup by PT Universal Pharmaceutical Industries; Paracetamol Drops, Paracetamol Syrup (mint) and Vipcol Syrup by PT AFI FARMA; Ambronol Syrup and DOK-1 MAX Syrup by Marion Biotech PVT. Ltd.
In October last year, many deaths after using cough syrups were reported in Gambia. The World Health Organization (WHO) warned that the deaths could be related to four products namely Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup, produced by Maiden Pharmaceuticals Limited of India.
Laboratory analysis of samples of each of the four products confirmed that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants. Indian authorities then investigated and asked Maiden to stop producing the syrups.