This strong message was delivered during a national online conference held on May 23, convened by the MoH to evaluate the implementation of the Prime Minister's Official Dispatch No.65/CD-TTg (dated May 15) and Directive No.13/CT-TTg (dated May 17). These directives focus on bolstering measures against smuggling, trade fraud, and counterfeit goods in the current climate.
Chairing the conference, Minister of Health Dao Hong Lan underscored the paramount importance of regulating pharmaceuticals, cosmetics, dietary supplements, and medical devices.
"This is a critically significant field directly impacting public health, demanding the synchronized engagement of the entire political apparatus," she stated.
Minister Dao Hong Lan affirmed that the MoH consistently identifies the management of healthcare-related products as a pivotal mission in disease prevention, treatment, and public healthcare.
"The Ministry of Health’s guiding principle is a resolute, zero-tolerance fight against the production, trade, and circulation of counterfeit goods within the healthcare sector. All violations must be rigorously prosecuted, with no prohibited zones and no exceptions," Minister Dao Hong Lan emphasized. She further declared that the MoH will hold accountable any individuals or organizations found culpable of lax management or of abetting or shielding illicit activities.
Addressing recent cases uncovered by police involving counterfeit milk, pharmaceuticals, and dietary supplements, the Health Minister opined that the high-profit margins in the pharmaceutical and dietary supplement markets make them susceptible to exploitation by unscrupulous entities.
"Many perpetrators have managed to evade authorities by exploiting legal loopholes or practical inadequacies in enforcement," she noted.
Furthermore, Minister Dao Hong Lan highlighted that the rapid expansion of e-commerce and social media has compounded the complexities of managing medical products, posing significant risks to public health and safety.
Elaborating on the root causes, the Minister suggested that a lack of synchronized and rigorous post-market surveillance at the local level has contributed to recent violations. Many products, once released onto the market, are not subjected to adequate inspection and monitoring.
"'Under the principle of 'localities decide, localities implement, localities are accountable,' many products falling under local jurisdiction have not been stringently controlled post-market entry in recent times," Minister Dao Hong Lan pointed out.
Currently, the MoH is concentrating on drafting two decrees: one delineating authority for implementation across two local government tiers, and another on the decentralization of power to localities. Concurrently, efforts are focused on amending and supplementing relevant legal documents, including the revised Law on Food Safety.
In response to concerns from various localities regarding a shortage of human resources for post-market surveillance, juxtaposed with the increasing sophistication of counterfeiters, Minister Dao Hong Lan announced that the MoH has petitioned relevant authorities to amend Decree No.15/2018/ND-CP, which details the implementation of certain articles of the Law on Food Safety.
Moreover, she urged localities to conduct more frequent inspections and examinations to combat counterfeit and imitation goods, rather than relying solely on designated action months. The Minister also stressed the importance of empowering citizens to detect and report instances of trade fraud and counterfeit production to the authorities.

"The public is currently confused, unsure which products are officially authorized and which are counterfeit or imitations. That’s why applying information technology, combined with greater accountability from relevant agencies, departments, and businesses, is essential to tackle the issue at its root. Otherwise, implementation will prove challenging," Minister Dao Hong Lan shared.
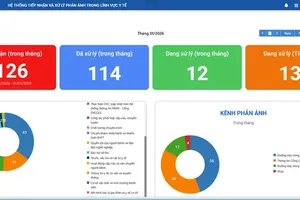
Statistics reveal that between 2020 and May 2025, the Food Safety Authority conducted inspections, examinations, and post-market surveillance at over 400 food establishments. It sanctioned 198 violating entities, imposing total fines of VND23.76 billion, and referred 31 cases involving the production and trade of counterfeit health supplements to police authorities for further investigation.