A representative from the Ministry, leaders of IVAC, based in the Central Province of Khanh Hoa, the Hanoi-based National Institute of Hygiene and Epidemiology and some related agencies yesterday announced at a press brief about the clinical trials’ phase 1 and 2 on human of the second Covid-19 vaccine.
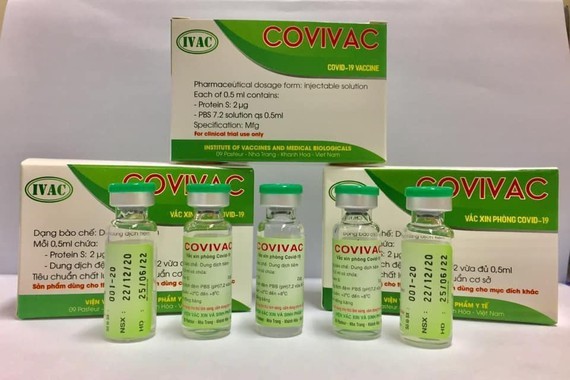
Covivac, a made-in-Vietnam Covid-19 vaccine developed by the Institute of Vaccines and Medical Biologicals (IVAC), has been sent to India and the US for trials on mice and trial results have showed a promising level of safety and efficacy, said Duong Huu Thai, head of the IVAC. Accordingly, IVAC has proposed the Ministry to allow the human trials.
It is scheduled that during the first phase on January 21 and 22, 125 volunteers will receive different doses. During the second phase, the vaccine will be injected on 250 volunteers.
Head of IVAC Thai added that the human trial will include three phases. However, the third phase will heavily depend on the first and the second phase’s results.