
The alert follows two major incidents involving the detection of substandard and undocumented drug products at pharmacies in the capital.
At An An Pharmacy in Ha Dong District, inspectors uncovered a batch of Theophylline Extended-release Tablets 200mg (lot 21127, manufactured February 2022, expiring February 2026), allegedly produced by Pharmacy Laboratories Plus. The packaging lacked all essential regulatory identifiers, including a drug registration number, import license, and importer information.
Laboratory tests revealed the product contained only 6.3 percent of the active ingredient Theophylline dosage stated on the label, a bronchodilator commonly used to treat asthma and chronic obstructive pulmonary disease (COPD).

Meanwhile, a broader investigation at Duc Anh Pharmacy, operated by Duc Anh Medical Equipment and Pharmaceutical Co., Ltd. in Dong Da District, uncovered a series of violations involving seven drug samples. Among them was DIAMICRON® MR 60mg (Gliclazide), lot 23F603, expiring April 2026, which failed to meet quality standards, containing only 70.83 percent of the labeled active ingredient.
More alarmingly, six additional products seized at the same location were found to be completely undocumented—lacking any drug registration number, import license, manufacturer, or importer identification. These included:
- Oseltamivir, lot M1164B01, manufactured March 2021, expired March
- Crestor 20mg (Rosuvastatin), lot A23237030, expiring April 2026
- Janumet 50/1000mg (Sitagliptin/Metformin), lot 24497505A, expiring July 2026
- Plavix (Clopidogrel), lot ELB04027, expiring May 2027
- NEXIUM® 40mg (Esomeprazole), lot 23H420, expiring September 2027
- Crestor 10mg (Rosuvastatin), lot A24236004, expiring July 2027
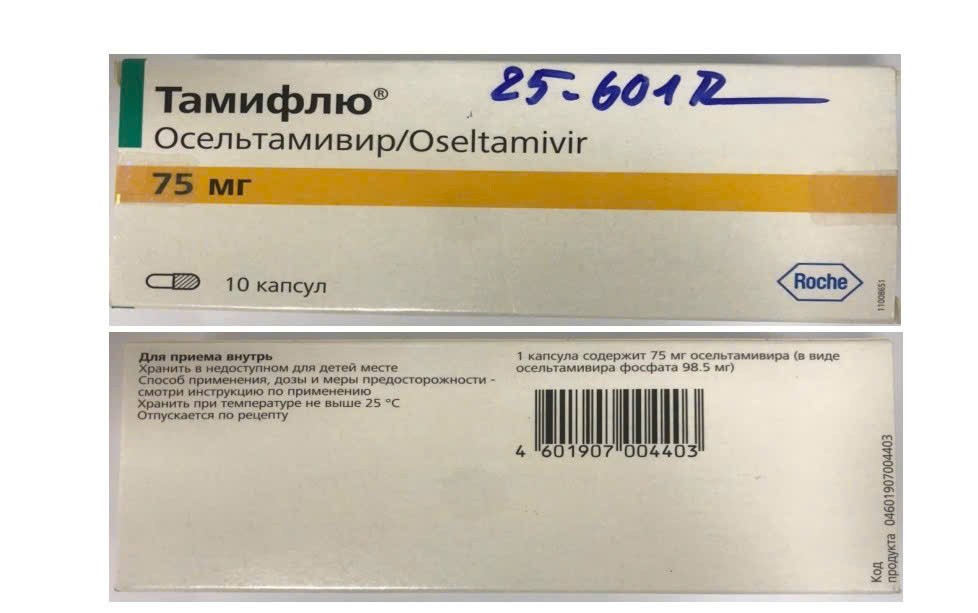
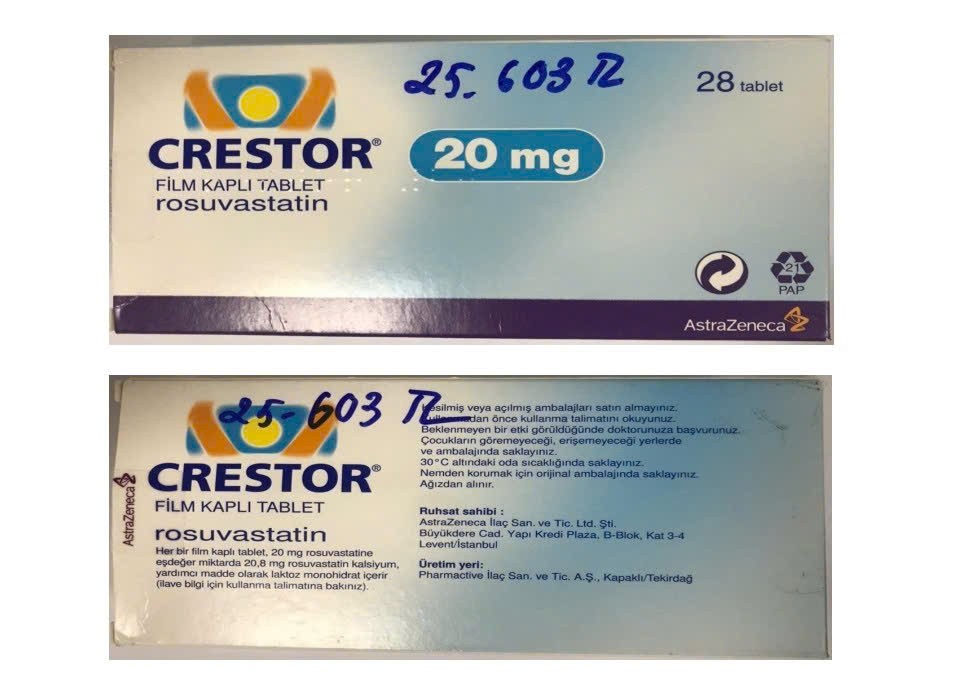

None of these products bore the required legal documentation, casting doubt on their origin, authenticity, and safety.
In light of these findings, the DAV has directed Hanoi’s Department of Health to coordinate with law enforcement, market surveillance units, and the city's Steering Committee for Anti-smuggling, Counterfeit Goods and Trade Fraud (Steering Committee 389) to launch comprehensive inspections of the pharmacies involved, identify the supply chains of the counterfeit products, and impose strict penalties. The outcomes must be reported to the DAV by June 2.
Simultaneously, health departments across all provinces and municipalities have been instructed to halt the circulation and use of these flagged products, issue warnings to pharmacies and healthcare institutions, and intensify public education campaigns. Citizens are urged to buy medicines only from licensed sources and to report any signs of counterfeit or untraceable drugs to the authorities immediately.
























