 |
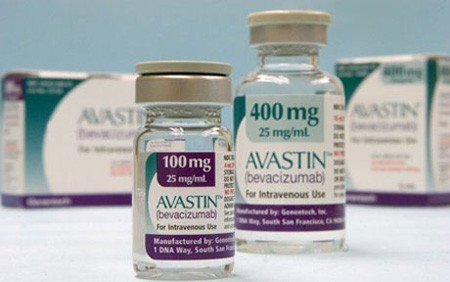
Four registration certificates of Avastin’s circulation still valid in Vietnam, drug watchdog says. |
Pakistani health authorities have been launching an investigation of Avastin, a cancer drug manufactured by the Swiss pharmaceutical company Roche after 12 patients went blind after being injected with the drug, the Drug Administration of Vietnam under the Ministry of Health announced through a review in Vietnam, four registration certificates of the drug’s circulation are still valid.
Avastin is indicated in the treatment of metastatic colorectal cancer, non-small cell lung cancer, advanced and/or metastatic renal cancer, glioblastoma/malignant glioma in stage IV approved by the World Health Organization, epithelial cancer of the ovary, fallopian tube and primary peritoneal cancer.
However, the drug is not used for intravitreal injection because these reactions can cause intraocular infection, endophthalmitis, and conjunctival hemorrhage. Some events have resulted in adverse events of varying severity, including permanent blindness.
The Drug Administration of Vietnam revealed that it has so far not received any reports of unwanted effects of the drug Avastin produced by Roche Pharmaceutical Company including patients’ vision loss after using the drug.