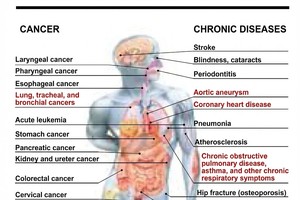
The recalled medications are because they contain a chemical that poses a potential cancer risk.
VAD requested to recall valsartan-containing medications made by Zheijang Huahai Pharmaceutical Co.,Ltd, Zhuhai Rundu Pharmaceutical Co.,Ltd, Zheijang Tianyu Pharmaceutical Co.,Ltd and Hetero Labs Ltd.
The recalled drugs include Valsartan160, with its registration No. VD-29714-18 made by Cuu Long Pharmaceutical Company; Cobidan 80, with its registration No. VD-22086-15 manufactured by BV Pharma company; drug Meyervasid M with its registration No.VD-30052-18; Mayervas 160, its registration No. VD-26480-17; Mayervas 80, its registration No. VD-26481-17; Mayervasid, its registration No. VD-26482-17; Meyervasid F, its registration No. VD-26483-17 made by Meyer – BPC company; and Valthotan F11m Coated Tablets 160mg “Standard”, its registration No. VN-17592-13 by Standard Chem & Pharm Co Company.
The health authority ordered above-mentioned enterprises to quickly work with distributors and importers to recall all drugs from retailers and wholesalers. They must report the task before September 30.