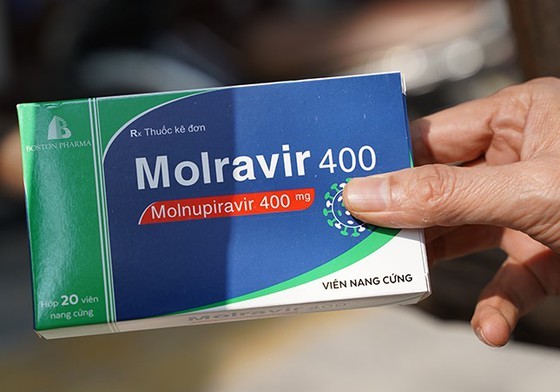
In its report to the Prime Minister, the Ministry said that Molnupiravir is a new drug, it is necessary to continue to monitor the quality, effectiveness, and safety of the drug during circulation. Currently, the drug has been licensed for conditional circulation by the Ministry of Health for three years and must be strictly controlled after licensing. People can only use Molnupiravir with a prescription from a doctor. Arbitrary use carries many risks due to unwanted effects of the drug as well as the creation of new strains of viruses.
In the UK, the USA, Japan and some countries where emergency use Molnupiravir has been approved, it can only be prescribed by doctors, registered nurses in advanced practice, and licensed physician assistants on legal prescription, said the Ministry of Health.
At the same time, the number of Covid-19 patients is increasing and the majority of patients with mild and moderate severity, are being treated and isolated at home. Presently, medical workers are overloaded with tasks; they have no time for prescribing the pill as per the regulation meanwhile, this drug is recommended to be used as early as five days from symptom onset or positive result.
To ensure easy access for patients to antiretroviral drugs to treat Covid-19 in the context of a high number of cases, the Ministry of Health proposed several contents on the dispensing of Molnupiravir. Specifically, for the distribution of free treatment drugs, localities and medical facilities can be assigned to purchase drugs to treat Covid-19, including Molnupiravir, which has been licensed for circulation in Vietnam according to regulations to promptly satisfy infirmaries’ needs.
Notably, in case people buy drugs themselves, pay for themselves at drug retailers, the person in charge of pharmacy expertise at the pharmacy or drugstore is allowed to prescribe for patients or buyers of antiretroviral drugs. Patients with Covid-19 can buy the pill if they have confirmation from medical facilities affirming that they are positive for SARS-CoV-2 ( including PCR or rapid test). Another way is that patients can record a clip of the process of performing the antibody test at home and send it to the person in charge at the pharmacy or drugstore to prove the positive test result.
Next, a person in charge of pharmacy expertise at the drug retail establishment, based on the test results, determines at least one patient's risk of severe disease. Drug purchaser or the patient must sign an agreement, which contains information about the patient, test date, test results, drug use according to the form attached with a copy of citizen identity card/identity card of patients.
The Ministry of Health also requires retail establishments that are pharmacies and drugstores to sell drugs, summarize and report on business results of Covid-19 treatment drugs by 5 p.m. daily and send them to the local health centers.
According to the Ministry of Health, this proposal only applies to oral antiviral drugs for Covid-19 treatment in areas, where the epidemic development is complicated while medical workers are overloaded with such various tasks that they are not possible to prescribe drugs according to regulations. Directors of departments of health in cities and provinces decide on the application of the plan based on the epidemic situation.