Vietnam is one of 15 countries involved in researching and testing this drug, with the trial completing on May 30. Among the 127 children participating worldwide, Vietnam has 20.
Notably, trial results show that after treatment, two children were able to walk, while others experienced improvements in motor function.
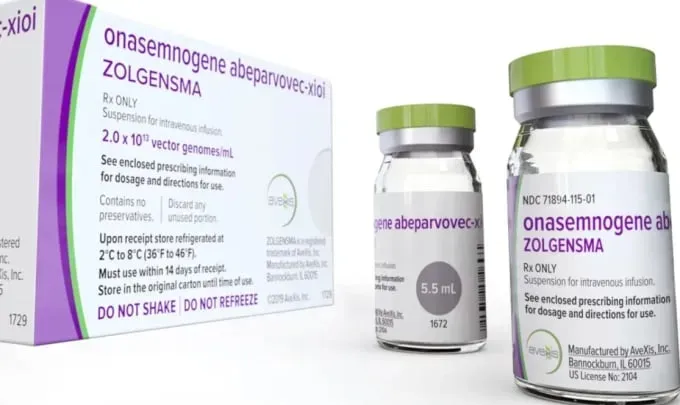
According to Dr. Vu Chi Dung, Director of the Center of Endocrinology, Metabolism, Genetics and Molecular Therapy at the Vietnam National Hospital of Pediatrics in Hanoi, the hospital has recently been involved in clinical trials for rare diseases, including spinal muscular atrophy (SMA), using drug Zolgensma developed by Novartis, a Swiss-American multinational pharmaceutical corporation.
Spinal Muscular Atrophy (SMA) is a genetic disorder that causes the degeneration of motor neurons, leading to muscle weakness and atrophy. It is a leading cause of death in infants due to respiratory muscle paralysis. The trial drug called Zolgensma, the world’s most expensive drug priced at over VND50 billion (nearly US$2 million) per dose, is a one-time intravenous infusion gene therapy that addresses the root cause of Spinal Muscular Atrophy (SMA) by replacing the faulty SMN1 gene with a functional copy.
Children who have completed the drug treatment will enter a five-year follow-up phase to continue evaluating the drug’s effectiveness.