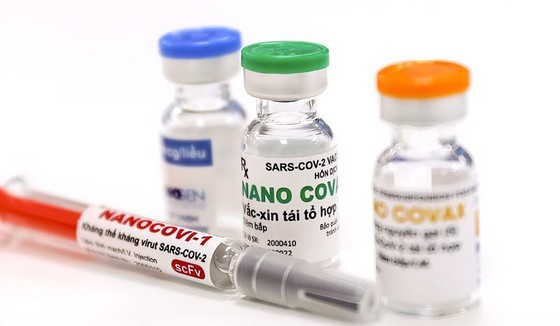
 Vietnam’s first homegrown Nano Covax vaccine
Vietnam’s first homegrown Nano Covax vaccine
Head of the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients under the Ministry of Health Professor Le Van Truyen said the council will give the vaccine serious consideration of emergency license grant in a cònerence on August 29.
In addition, the vaccine developer, Ho Chi Minh City-based Nanogen Biopharmaceuticals Company, should submit a more perfect registration document of the vaccine to the Drug Administration of Vietnam
Chairman of the National Committee for Ethics in Biomedicine Research Professor Truong Viet Dung affirmed only the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients can grant an emergency license for the vaccine while the National Committee for Ethics in Biomedicine Research has just approved its the results from Nano Covax's phase 3a clinical trials. However, the Nano Covax's phase 3a clinical trials’ results is an important factor for the decision of emergency license grant.
According to the Ministry’s regulation, the head of the Drug Administration of Vietnam will issue a certificate of registration for circulation of the vaccine within 20 days from the date of receipt of a complete application for a vaccine registration certificate.
The licensing will be based on circular 11 on the Ministry of Health’s guidelines for registration of circulation of Covid-19 vaccines in case of emergency, which has just been issued on August 19.
Accordingly, the vaccine is eligible for an emergency license while it is in clinical trials and the results of the phase 3a mid-term trial on safety and protective efficacy of the vaccine are based on the vaccine’s immunogenicity data. At the same time, the licensing will be based on the opinion of the National Ethics Council in biomedical research and the advice of the Advisory Council for the issuance of circulation registration certificates for drugs and medicinal ingredients with the recommendations of the World Health Organization.
The results of the phase 3a mid-term evaluation have shown that the Nano Covax vaccine had a seroconversion rate of up to 99.2 percent on the 42nd day. Therefore, the research team concluded that the mean multi-concentration average antibody levels increased 218.93 times including stages 2 and 3a after 42 days of vaccination. With this result, the Covid-19 Nano Covax vaccine meets the requirements for immunogenicity and continued to perform the phase 3b study on 12,000 volunteers.
In addition to consideration of the application for the Nano Covax vaccine, the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients will review the application for an emergency license of the Hayat-Vax vaccine manufactured in the United Arab Emirates (UAE) on August 29.
This is a vaccine that is produced basing on transferred technology with Sinopharm of China. Previously, Vimedimex Medical and Pharmaceutical Joint Stock Company proposed to the Prime Minister approve the vaccine as well as direct the Ministry of Health to quickly appraise the application for emergency registration of Covid-19 Hayat-Vax vaccine for the fight against Covid-19.