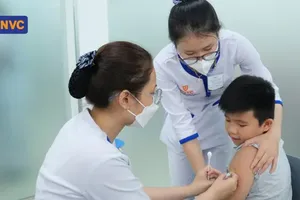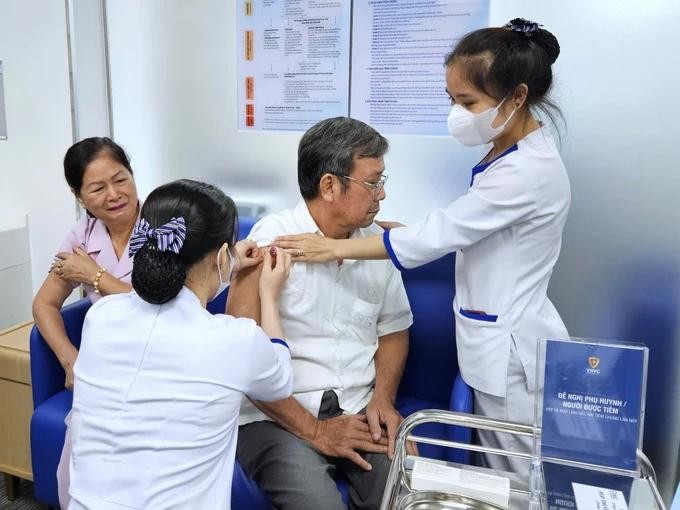
The Vietnam Vaccine Company (VNVC) yesterday officially introduced a new vaccine against 23 different strains of pneumococcal bacteria that cause serious diseases such as meningitis, sepsis, pneumonia with sepsis and community-acquired pneumonia.
The pneumococcal vaccine 23, developed by the American pharmaceutical firm MSD, is now accessible in almost 50 countries globally, marking the first occasion for the Vietnamese population to receive this vaccination.
This vaccine is designed for individuals aged two and older, with particular efficacy noted among older adults who have pre-existing health conditions. It is especially beneficial for those who have experienced diminished respiratory capacity following a Covid-19 infection, which may manifest as persistent cough, hoarseness, breathing difficulties, fatigue, or bronchitis.
Additionally, it targets high-risk populations, including individuals over the age of 65 and those with immunodeficiencies such as HIV, cancer, spleen dysfunction, splenectomy, sickle cell disease, chronic kidney failure, nephrotic syndrome, and those undergoing treatment with immunosuppressive medications.
Head Vu Thi Thu Ha of VNVC’s supply department said that in the past 8 years, VNVC and vaccine companies have brought 10 new vaccines to Vietnam, helping to minimize vaccine shortages, especially helping to prevent many dangerous infectious diseases for children and adults.
In its role as a strategic partner to MSD Pharmaceutical Group, VNVC has proactively placed early orders and collaborated closely with MSD Vietnam to ensure the availability of this crucial vaccine for the public. At present, the pneumococcal vaccine 23 is readily accessible at nearly 200 VNVC vaccination centers across the country.
These vaccines are securely stored in a network of hundreds of cold storage facilities that comply with international Good Storage Practices (GSP) standards, making them prepared for widespread administration to children aged two and older, as well as to adults and elderly individuals with pre-existing health conditions.
Statistics indicate that in 2021, approximately 9.8 million individuals were affected by pneumococcal diseases, with a mortality risk reaching as high as 25 percent despite appropriate treatment and antibiotic administration. The World Health Organization reports that children and the elderly in developing nations are particularly vulnerable, with an estimated annual death toll of 1 million children attributed to pneumococcal infections.