He made the statement at a forum discussing the achievements in veterinary vaccine research and application in Vietnam.
Among these, 12 facilities are dedicated to producing veterinary vaccines, with each factory requiring an investment of approximately US$30 million-$40 million.
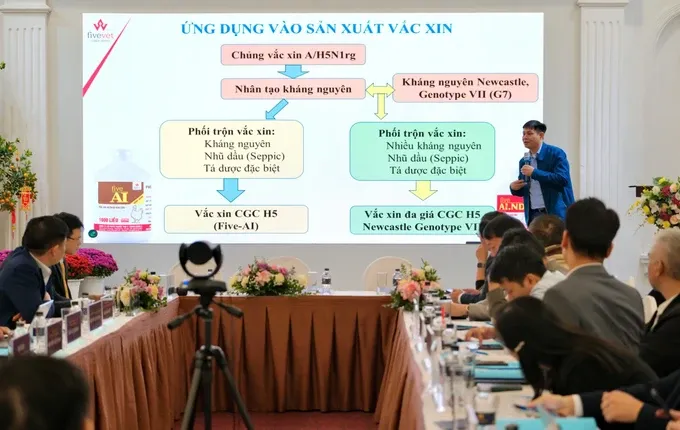
Companies such as Vaksindo, Hanvet, Navetco, and Dabaco have successfully established self-sufficiency in the production and supply of 218 types of domestic vaccines, in addition to importing 340 other vaccine types to meet the disease prevention needs of livestock and poultry in the country.
In terms of domestic vaccine production, by 2024, these companies have manufactured and supplied 739 million doses of avian influenza vaccine, over 46 million doses of foot-and-mouth disease vaccine, 34 million doses of blue ear disease vaccine, nearly 2 million doses of lumpy skin disease vaccine, and notably, 5.9 million doses of African swine fever vaccine.
This marks a significant advancement, affirming Vietnam's capability for self-reliance in the veterinary vaccine sector.